To run a successful business, you want to make and distribute products that are at the right quality standard and that comply with the regulations of the countries of sale. I guess nobody will argue with that. But what do “Quality and Compliance” mean, in practice?
They are often separated under different functions inside the same company. Why?
Let’s look at these 2 concepts in the context of manufacturing products and importing them.
“Quality with a capitalized Q” includes compliance
For example, quality management systems standards such as ISO 9001 and, to a greater extent, ISO 13485, emphasize the need to collect both customer requirements and regulatory requirements.
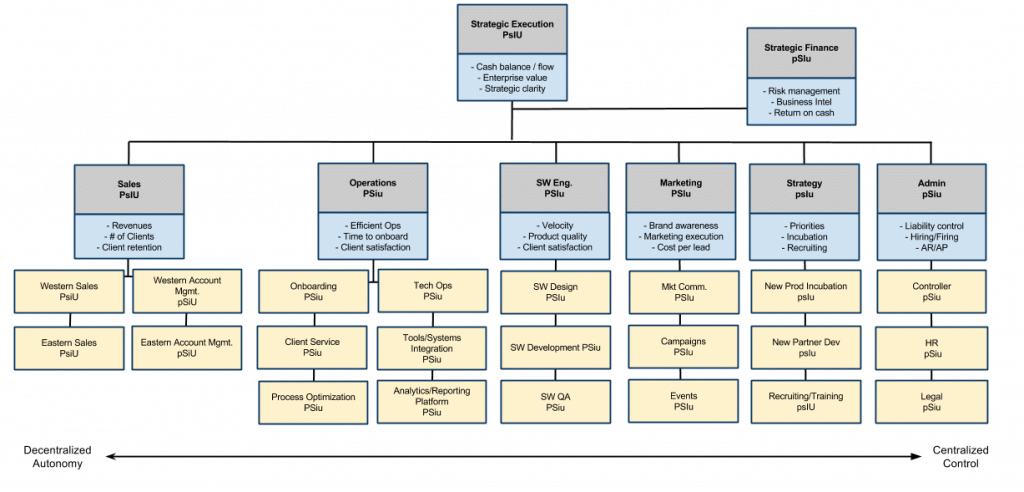
I drew a diagram that makes this clear:
In this view, there is no real difference. “Quality with a big Q” does look at regulatory requirements.
However, what one might call “quality with a small q” focuses only on making products up to a specific standard. Many companies hold this view.
And they often evaluate results in terms of “how good is our quality?” by checking if they met their target standard “in most shipments”, “on most products”, “by a nice margin vs. just getting by”.
In other words, it is not black or white. Performance is somewhere along a continuum, and there is room for improvement.
In some cases, managers wonder if they over-shot what their customers really want. In an effort to contain or even to cut costs, they are sometimes ready to target a lower standard.
All this is not true of compliance, as I will cover in the next point. Hence the need to distinguish these 2 functions in many companies.
Compliance includes product quality but often responds to a different logic
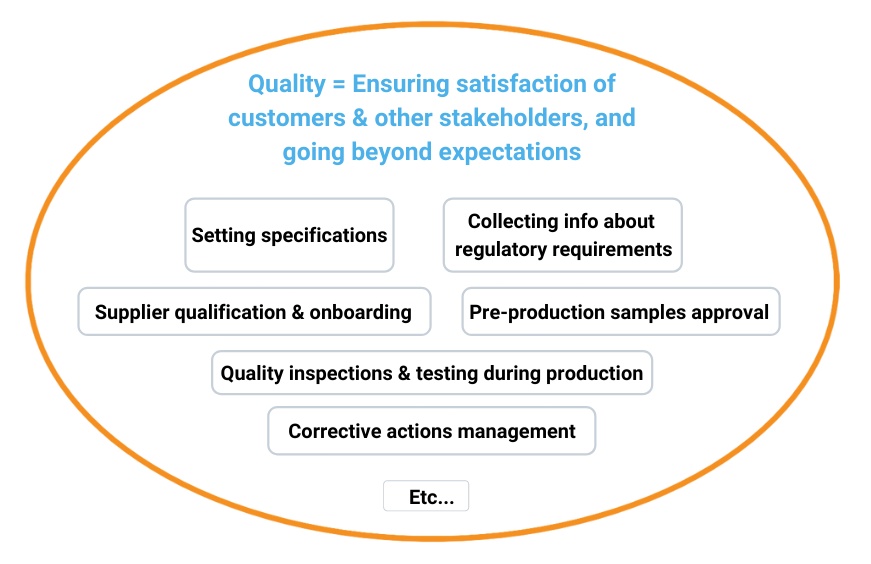
Compliance is certainly not “separate”. It requires many quality activities to be undertaken, as shown below:
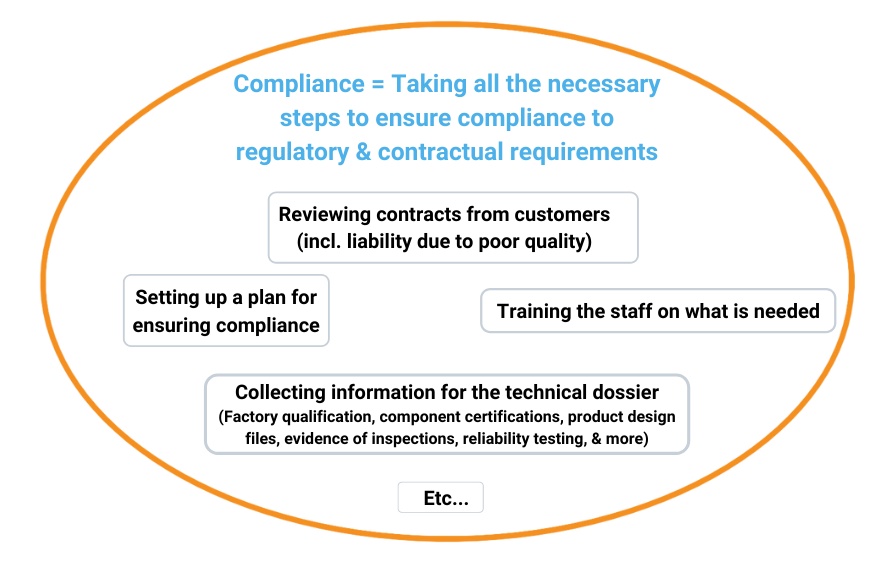
However, as I suggested above, it follows a different logic. When market surveillance authorities (or, worse, a court of justice) look into the steps a manufacturer or importer took to assure a product complies with applicable regulations, their conclusion will be “yes” or “no”. It is far more “black or white”.
The stakes are different. Instead of “do consumers get a nice first impression when they see the finishing of the product?”, the question is more likely to be “can you demonstrate that this product is safe?”
Having said that, of course, quality does help with product safety and with compliance. So does reliability. See Why Product Safety, Quality, and Reliability Are Tightly Linked.
Many companies separate the management of both
Quality is often managed close to manufacturing. With inputs from product designers and marketing, quality engineers prepare specifications, quality control technicians inspect production batches, quality managers push suppliers to implement corrective actions, etc.
And that makes sense. Being close to manufacturers certainly helps follow up.
Compliance, in contrast, is often managed at the headquarters. Because, in a way, it is a legal function.
Admin and legal functions tend to be centralized because high control is needed and they generally don’t need to be located very close to the action.
All this is consistent with the suggestions of Lex Sysney. Here is his general representation of that idea (click to enlarge):
(Source)
One final point on the topic…
In many cases, compliance is an “internal customer” of quality. The risk reviews, the data collection, the inspection & testing reports, the correction and corrective action plans, etc. all need to be collected in the product technical files.
*****
Do you have any thoughts or questions on the topic of quality compliance? Let me know by leaving a comment or contacting me, please.