What does quality management consist of?
Juran wrote about this first in 1986, and he suggested 3 types of activities that make up the “Juran trilogy”:
- Planning
- Quality control
- Improvement
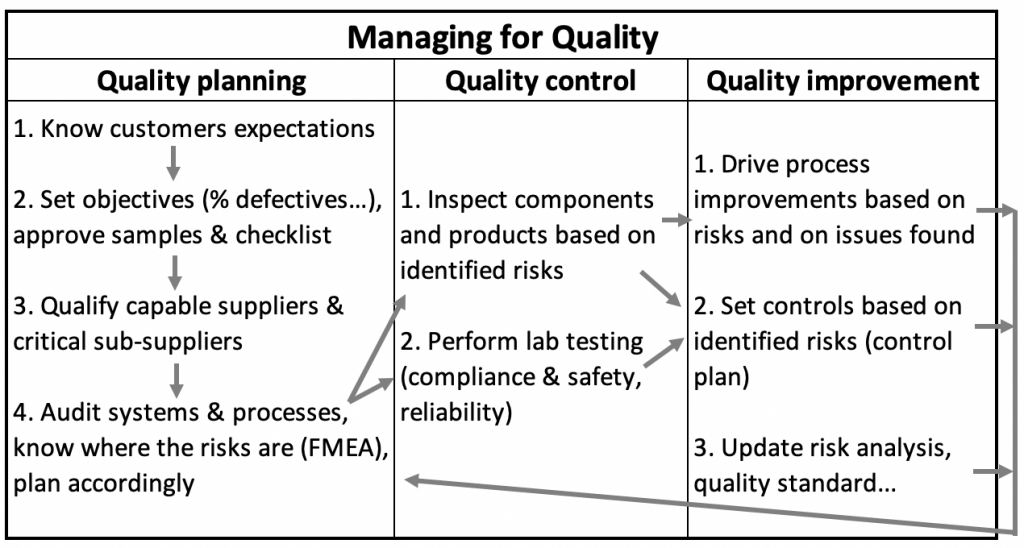
How do quality planning, control, and improvement work together?
I kept the same basic framework idea and took some liberties with the content of the table.
Here is what importers need to consider when setting up a quality assurance program for the products they manufacture in China/Vietnam.
Let’s go through these elements one by one, starting with ‘Quality Planning’
First, a word of warning.
I will assume you can assign the resources necessary to ensure you reach your objectives with a high confidence. What I wrote here is not a good fit for Amazon sellers who move small volumes of off-the-shelf products, for example.
1. Know customers’ expectations
What market(s) do you sell to, in what distribution channel(s)? Knowing your customers and end-users will help you answer these questions:
- What is most important (“critical to quality”)? What is a must-have? What is a nice-to-have?
- What are issues they don’t want to see? The battery in a widget can’t get recharged, the thickness of the steel used for construction is below tolerance, small color variations on different units that will all be shown on the same shelves…
- What proportion of defective products (remember, you need to define what is defective in their eyes) can they tolerate?
2. Set objectives and approve samples & checklist
Based on what the above questions led you to discover, the best practice is to:
- Draft a clear and specific specification sheet, including defining the most common potential defects
- Set AQL limits
- Approve sample(s) that have the right touch & feel, and if needed also boundary samples (to illustrate the limit of acceptance)
- Have the supplier(s) confirm all these in writing (if possible under the frame of a legally enforceable agreement)
3. Qualify capable suppliers & critical sub-suppliers
If you are setting up the supply chain for a new product, this playbook will go a long way in helping you reach your objectives:
- Identify the critical components — those with high value and/or those associated with a high risk (and that usually means the highly customized components make that list) — or processes (typically surface treatment such as paint, anodizing, plating…)
- Use your documented objectives and a relevant auditing checklist to qualify those components’ suppliers
- In parallel and the same way, qualify the final processing & assembly manufacturer
Note: meeting your company’s target price and your quality objectives might be impossible. That’s why planning, and in particular documenting the objectives, is so important. Knowing this information early in the process is much better than after a production batch is made…
4. Audit systems & processes, know where the risks are, plan accordingly
As part of the supplier qualification process, you will need to audit their quality systems and maybe also their processes. You will know where the major gaps are, and you will have an idea of the highest risks for your project.
A very good tool here is also the process FMEA. It doesn’t start from a checklist, but from an estimate of the major potential failure modes (in other words, from a risk assessment).
When and where to do ‘Quality Control’
Based on the above information, deciding on the next step (the second part of Juran’s trilogy which is when and where to do quality control) can be based on data.
1. Inspect components and products based on identified risks
The critical components may be checked at the sub-suppliers’ facilities (if authorized), or in the assembler’s facility. And the production can be inspected at several stages. It all depends on the main risks that were identified.
If one process is particularly fraught with uncertainty, for example plating, it can make sense to be present during the pilot run and then during production.
2. Laboratory testing (compliance & safety, reliability)
This is often a complement to the QC inspections. Inspectors can pick samples at random and send them to a lab for testing.
People often think of compliance testing (to make sure you can sell the batch on your market with low legal risks) but much less often of reliability testing (to make sure your products won’t fail too early in the hands of your customers).
Ongoing ‘Quality Improvement’
Now, let’s examine the final part of the trilogy:
1. Drive process improvements based on risks and on issues found
Some issues are identified (in the audits, the FMEA, the inspections, etc.) and can lead to:
- Corrections – fixing an issue in the short term (for example, to make sure a batch can be shipped out at acceptable quality and on time) — this is often done but doesn’t improve systems and processes in the long run.
- Corrective actions – understanding a problem in-depth and addressing at least 1 root cause so that problem doesn’t come back in the future — this is very powerful and you probably need to do more of those. A good corrective action plan framework is 8D.
- Preventive actions – addressing a risk before anything bad happens — this is also powerful, and can also be done with the 8D approach.
Needless to say, working closely with suppliers is a must. They need to be cooperative and open.
2. Set controls based on identified risks
If a manufacturing process lacks a structured way of ensuring good outputs, don’t take several isolated actions.
Taking a holistic approach and setting up a process control plan (and its close cousin, the preventive maintenance plan, if equipment is a common source of issues) will usually lead to faster and more sustainable improvements.
3. Update the quality standard, risk analysis, etc. over time
The planning is not done once and for all. New information surfaces every week. Some suppliers will change, some new defects will come up, some end users will complain, and so forth.
Keep a list of the sources of high risks you have identified and have not sufficiently mitigated. Then, adapt your plan for quality control and your focus for improvements.
How does it work as a system?
It is not a linear sequence of steps (1, 2, 3…). It is a loop, as indicated by the arrows if you consider this plan again.
The overall logic goes like this:
- Make sure your objectives are clear and use that to qualify suppliers.
- As suppliers get audited, you discover risks, which you can address.
- Plan for the most appropriate way to inspect and test components, processes, and finished products.
- The findings point to further gaps, allowing you to refine the overall plan.
- Address these gaps with isolated corrective action requests.
- When possible, adopt a more system-wide approach, for example with a control plan.
- At regular intervals, review your objectives and update your plan.
*****
This framework is nothing new to some companies, especially in certain industries (e.g. automotive, pharmaceutical). What I found out over the years is that most importers operate without a framework. They make decisions without data.
Think about it. Managing procurement and quality in China, Vietnam, India, Malaysia, etc. consists, in large part, of risk management. Do you think those finance professionals who make risk vs. reward decisions operate without a framework and without data? Of course, they don’t. And neither should you.
Sofeast: Quality Assurance In China Or Vietnam For Beginners [eBook]
This free eBook shows importers who are new to outsourcing production to China or Vietnam the five key foundations of a proven Quality Assurance strategy, and also shows you some common traps that importers fall into and how to avoid or overcome them in order to get the best possible production results.
Ready to get your copy? Hit the button below: